- Home›
- Medical Devices›
- Diagnostic Detection Devices›
- Rapid Test Kits (RDT)›
- Hepatitis Test Kits
Hepatitis Test Kits
Type
Specimen
Form
Packaging
What are Hepatitis Test Kits?
Hepatitis Test Kits are medical diagnostic tools used to aid in the diagnosis of hepatitis infection. There are different types of hepatitis, including hepatitis A, B, C, D, and E, and each type requires a specific, specialized test kit. Hepatitis is liver inflammation that is commonly caused by a viral infection.
Hepatitis diagnostic test kits are designed to provide quick and accurate results. They can be used in many healthcare settings, including hospitals and clinics. The hepatitis screening testing kits are available as individual strips, cassettes, or as multi-panel cassettes. Each of the tests utilizes an immunochromatic assay, which is designed to provide quick and accurate results. They can be used in many healthcare settings, including hospitals and clinics.
The hepatitis screening and diagnostic test kits utilize rapid chromatographic immunoassays, which allow for the qualitative detection of the antigens or antibodies specific to the test type.
AdvaCare Pharma is the supplier of Hepatitis Test Kits, which are manufactured in ISO and CE-certified facilities in India, China, and the USA. Every facility is regularly inspected to ensure they are compliant with healthcare standards for quality and safety.
Product Specifications
Type
Specimen
Form
(HAV) Hepatitis A Antibody IgM Test Kit
(HAV) Hepatitis A Antibody IgM Test Kit is a qualitative diagnostic tool used to detect the presence of IgM antibodies. It is designed to be used with whole blood or serum/plasma specimen.
The HAV test kit is available as a strip or a cassette with a strip inside. The test strip is an immunoassay, which has reagents that are specific to hepatitis A virus IgM. Any antibodies present will bind and react, which will show a visible sign, which indicates a positive result.
(HBsAb) Hepatitis B Surface Antibody Test Kit
(HBsAb) Hepatitis B Surface Antibody Test Kit is a rapid qualitative test used to detect any present antibodies against the hepatitis B virus surface antigen. It is designed to be used with whole blood or serum/plasma specimen.
The HBsAb test kit is available as a strip or a cassette with a strip inside. The test strip is an immunoassay, which has reagents that are specific to hepatitis A virus IgM. Any antibodies present will bind and react, which will show a visible sign, which indicates a positive result.
(HBsAg) Hepatitis B Surface Antigen Test Kit
(HBsAg) Hepatitis B Surface Antigen Test Kit is a rapid qualitative assessment designed to detect hepatitis B virus surface antigen (HBsAg) in whole blood or serum/plasma.
The HBsAg test kit is available as a strip or a cassette with a strip contained inside. The strip is a lateral flow immunoassay, which will show a visible signal if there is the presence of HBsAg.
(HBV) Hepatitis B Test Panel (5 in One) Kit
(HBV) Hepatitis B Test Panel (5 in One) Kit is a rapid qualitative assessment that combines five different tests into one comprehensive testing device. It is designed to be used with whole blood or serum/plasma specimen.
The multi-panel cassette includes testing strips for the HBV surface antigen (HBsAg), HBV surface antibody (anti-HBs), HBV core antibody total (anti-HBc), HBV core antibody IgM (IgM anti-HBc), and hepatitis B E antigen (HBeAg).
(HBC) Hepatitis C Antibody Test Kit
(HBC) Hepatitis C Antibody Test Kit is a qualitative diagnostic tool used to detect the presence of IgM antibodies specific to hepatitis C. It is designed to be used with whole blood or serum/plasma specimen.
The HBC test kit is available as a strip or a cassette with a strip inside. The test strip is an immunoassay, which has reagents that are specific to the hepatitis viral IgM. Any antibodies present will bind and react, which will show a visible sign, which indicates a positive result.
(HEV) Hepatitis E Antibody IgM Test Kit
(HEV) Hepatitis E Antibody IgM Test Kit is a lateral flow chromatographic immunoassay that can be used for qualitative assessment of the presence of Hepatitis E antibodies.
The HEV test is available as a strip or a cassette with a strip inside. The strip is able to detect anti-HEV IgM within the sample. It is designed to be used with whole blood or serum/plasma specimen.
Whole Blood
Whole Blood specimen is needed for this type of hepatitis test. Collect blood using standard venipuncture (2 drops) or fingerstick (50 μL) techniques. This specimen is transferred (after centrifugation, in the case of serum/plasma samples) with a capillary tube or dropper to the assigned area of the cassette, where a drop of the corresponding buffer dilution will be added immediately afterward to proceed with the test.
Serum/Plasma
Serum/Plasma specimen is needed for this type of hepatitis test. After centrifugation, serum or plasma is carefully transferred to the designated area of the cassette using a capillary tube or dropper. Subsequently, a drop of the appropriate buffer dilution is promptly added to the cassette, enabling the test to commence.
Proper handling and precise execution of the transfer and buffer addition steps are crucial for the successful completion of the test.
Cassette
Cassette is a box made of plastic, which has a test strip inside of it, a designated area for sample placement, and a window to view the results of the test.
The strip inside of the testing cassette utilizes an immunochromatographic assay, which will display the results as positive, negative, or invalid.
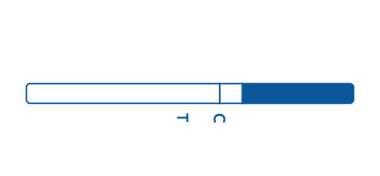
Strip
Strip is a thin piece of plastic containing reactive agents. Each strip features an area specific for sample application and an area in which the test results appear.
The strip features an immunochromatographic assay-based test, or a lateral flow assay, which is used for qualitative detection.
To use the strip, the arrow end of the strip should be dropped into the specimen sample into the arrow end of the strip. The test results will appear as positive, negative, or invalid.
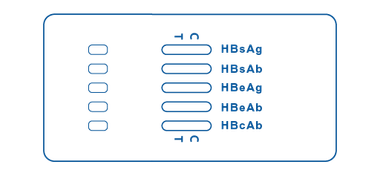
Multi-Panel Cassette
Multi-Panel Cassette consists of a wider cassette that contains 5 test strips inside, one for each HBV marker (HBsAg, HBsAb, HBeAg, HBeAb, and HBcAb). It has different areas for sample placement and windows in which the lines will display as a positive, negative, or invalid test result for each one.
Why are we a top Hepatitis Test Kits manufacturer?
AdvaCare Pharma is a leading manufacturer of Hepatitis Test Kits, a product within the AccuQuik™ brand of diagnostic detection devices. Our production facilities are held to stringent CE and ISO standards. As a manufacturer of rapid test kits, AdvaCare Pharma partners with an extensive global network of institutions, including hospitals, pharmacies, distributors, and more.
For 20 years, AdvaCare Pharma has been dedicated to providing premium and affordable pharmaceutical products for an ever-changing global community. We prioritize our partners and pride ourselves on building lasting and mutually beneficial relationships.
Uses
How should Hepatitis Test Kits be used?
The process for using Hepatitis Test Kits depends on the specific type of Hepatitis and test being conducted. Generally, the steps are as follows:
- After checking that the kit is within its expiration date and properly stored, the user follows instructions to collect the appropriate specimen, whether it is whole blood, serum, or plasma. This may involve venipuncture or fingerstick techniques to obtain blood samples.
- Once the specimen is collected, it is carefully administered to the designated area on the test kit, which could be a cassette, strip, or multi-panel cassette depending on the type of kit.
- The user then adds a specified amount of buffer solution to initiate the test.
- After the prescribed waiting period of 15-20 minutes, the results are interpreted based on the appearance of lines or other indicators on the test device, following the guidelines provided in the kit's instructions. It is of the utmost importance to adhere to the instructions precisely to guarantee accurate results.
How should the test kits be stored?
These kits should be stored at room temperature in a dry location, away from direct sunlight, heat sources, and moisture. Exposure to extreme temperatures or humidity can compromise the integrity of the testing components and lead to inaccurate results.
Check the expiration date before use and avoid using any kits that have expired. Damaged or compromised kits should not be used, as they may yield unreliable results. When you follow these storage guidelines, it preserves the quality of the test kits.
After use, how should the Hepatitis Test Kits be disposed of?
Disposal of Hepatitis Test Kits and related materials must be handled in accordance with local regulations and guidelines for biohazardous waste.
After conducting the tests and interpreting the results, any used test components, including used cassettes, strips, or multi-panel cassettes, as well as any leftover specimens, are considered biohazardous waste due to potential exposure to bloodborne pathogens. These materials should be carefully collected and disposed of in designated biohazard waste containers.
Follow established protocols for biohazard waste disposal to minimize the risk of contamination and guarantee the safety of individuals handling the waste and the environment. By adhering disposal procedures, healthcare facilities and testing sites can mitigate the potential hazards associated with handling infectious materials and encourage a safe testing environment.
FAQs
What is the difference between the Hepatitis Test Kits?
Different types of tests can be used to detect different components of the specified strain of the hepatitis virus.
The (HAV) hepatitis A antibody IgM test kit is designed to detect the presence of IgM antibodies due to the hepatitis A virus. These antibody testing kits can confirm an individual's immunity to the virus. They may also be used to check whether a vaccination was successful.
The (HBsAg) hepatitis B surface antigen test kit is designed to detect the hepatitis B virus surface antigen (HBsAg). Since it can detect the virus, it can be used to diagnose an acute or chronic infection.
The (HBV) hepatitis B test panel (5 in one) kit is a combination test that is able to detect the surface antigen (HBsAg) and any present antibodies (anti-HBs, anti-HBc, IgM anti-HBc). This offers a more comprehensive view of a patient's current state of infection.
The (HCV) hepatitis C antibody test kit is used to detect the presence of antibodies against the Hepatitis C virus, which can indicate a past or current Hepatitis C infection. Similarly, the (HEV) hepatitis E antibody IgM test kit can detect the presence of IgM antibodies associated with the hepatitis E virus. Its presence can indicate a recent or acute hepatitis E infection.
How long does it take to get the results?
The hepatitis rapid testing kits take about 15-20 minutes to show a result.
Do your Hepatitis Test Kits meet CE certification requirements?
Certainly, our medical devices meet CE certification standards, ensuring compliance with European Union regulations. Furthermore, our manufacturing protocols strictly adhere to ISO standards to maintain high-quality standards across all products.
Can I receive assistance with regulatory matters for importing your Hepatitis Test Kits?
Yes, we extend regulatory support and guidance to our distributors, aiding in adherence to local regulations and facilitating the smooth importation of our medical devices. Backed by our proficient Regulatory Affairs Department, comprising experts such as pharmacists, biomedical engineers, QA professionals, and documentation specialists, we ensure a seamless product registration process.
Are your Hepatitis Test Kits suitable for sale to medical institutions?
Our medical devices are suitable for distribution in labs, hospitals, clinics, pharmacies and other healthcare facilities, where they can be used to provide high-quality patient care. Our efficient distributor verification process makes it easy to get started. Contact our International Sales Department for more information about the distribution of our medical devices in your market.
You might be interested in...
Why AdvaCare Pharma?
As an industry leader, we are aware of our responsibility to provide affordable and sustainable solutions to improve healthcare worldwide.











